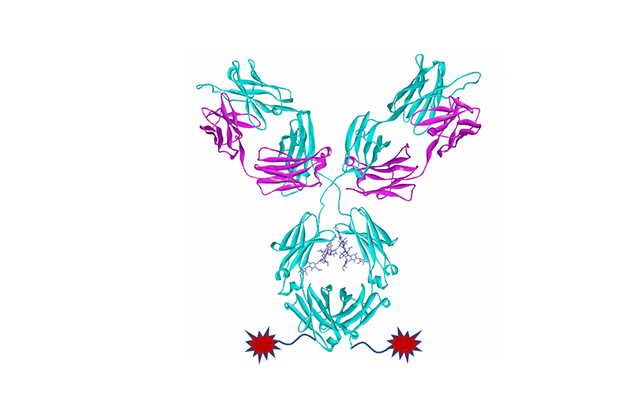
Glycosite-specific conjugation technology for ADCs, developed by GlycanLink, can specifically conjugate drug payloads to Asn297 N-glycan of IgG Fc, involved in two generations, which are GL-OligosacLinkTM and GL-DisacLinkTM.
- GL-OligosacLinkTM——The first generation of "two enzymes & three steps" ADC preparation technology
- ADCs with DAR value of 4/8/12 can be quickly achieved by specific enzyme-catalyzed methods. ADCs prepared by this method remain ADCC activity and FcRn functions of antibodies, and have excellent in vivo activity, pharmacokinetic activity, and safety.
- GL-DisacLinkTM——The second generation of "one enzyme & one step" ADC preparation technology
- Using disaccharide substrates developed by GlycanLink, ADCs can be achieved by a one-step reaction catalyzed by specific enzymes. The reaction is simple and efficient, and does not rely on bioorthogonal reactions. Compared with similar competitors, ADCs prepared by this method can reduce immunocytotoxicity and maintain the FcRn function. In addition, due to their good hydrophilicity and stability, these ADCs have significantly excellent in vivo activity, pharmacokinetic activity, and safety.
- GL-DisacLinkTM——The new generation of "one enzyme & two steps" ADC preparation technology
- Using azide bisaccharide substrates developed by GlycanLink, glycan-modified antibodies with linkers are prepared under the catalysis of specific enzymes, and then through bioorthogonal reactions, a variety of linkers and drug payloads can be applied to obtain ADCs with a wider range of DAR values.